More than 500,000 bottles of the blood pressure medicine prazosin hydrochloride have been recalled by pharmaceutical companies, according to the U.S. Food and Drug Administration, due to fears that the pill may contain a chemical that causes cancer.
The FDA reports that early this month, more than 580,000 bottles of different strengths of prazosine capsules were voluntarily recalled nationally by Teva Pharmaceuticals USA, based in New Jersey, and medicine distributor Amerisource Health Services.
Join our WhatsApp community

To help reduce blood pressure, doctors administer the blood pressure medicine prazosin. Additionally, post-traumatic stress disorder-related nightmares and other sleep disorders are occasionally treated with it.
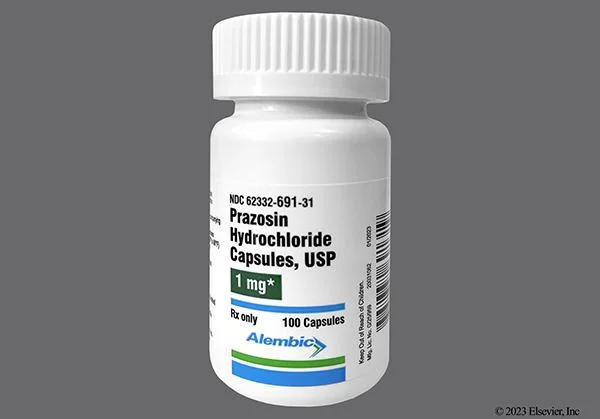
The FDA further stated in enforcement orders posted online that it has given the affected lots of the drug a Class II risk classification because some of the recalled medication may have nitrosamine impurities that are considered to have the potential to induce cancer.
Join our WhatsApp community

N-nitrosamine impurities are a type of possibly carcinogenic substances that might develop during the production or storage of a medication, according to the FDA.
Join Our Social Media Channels:
WhatsApp: NaijaEyes
Facebook: NaijaEyes
Twitter: NaijaEyes
Instagram: NaijaEyes
TikTok: NaijaEyes